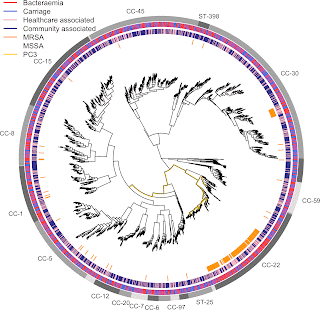
Staphylococcus aureus is a leading cause of infectious disease deaths in all countries, with bloodstream infection leading to sepsis a major concern. This new study, published in November in Microbial Genomics, reports genes and genetic variants in Staph. aureus associated severe disease vs asymptomatic carriage, and healthcare vs community carriage.
Our genome-wide association study of 2000 bacterial genomes showed that antibiotic resistance in Staph. aureus is associated with severe disease and the hospital environment:
- A mutation conferring trimethoprim resistance (dfrB F99Y) and the presence of a gene conferring methicillin resistance (mecA) were both associated with bloodstream infection vs asymptomatic nose carriage.
- Separately, we demonstrated that a mutation conferring fluoroquinolone resistance (gyrA L84S) and variation in a gene involved in resistance to multiple antibiotics (prsA) were preferentially associated with healthcare-associated carriage vs community-acquired carriage.
The implication – that antibiotic resistance genes may provide survival advantages which mechanistically contribute to the development of disease – is important in the face of the continued global rise of antibiotic resistance.
We were also able to shed light on a controversy as to whether different strains of Staph. aureus differ in their propensity to cause severe disease. Interest in this question dates back decades in the literature, and contradictory studies, often based on modest sample sizes, have reached different conclusions. Our comparatively large study, using a whole-genome method that we previously published in Nature Microbiology, found that all strains of Staph. aureus are equally likely to cause severe disease vs asymptomatic carriage.